Researchers in the lab of Stephen Fesik, Orrin H. Ingram II Chair in Cancer Research, have added BI-0474 as the second molecule co-discovered by Vanderbilt to the open science portal opnMe.com, an initiative being driven by biopharmaceutical company Boehringer Ingelheim.
BI-0474 is an irreversible covalent inhibitor of G12C mutated KRAS, in which a glycine amino acid of the protein is replaced by a cysteine. It holds the promise of blocking the cancer-causing functions of this mutation, which is one of the most common found in KRAS. KRAS is one of the most commonly mutated proteins in cancer, driving 32 percent of lung cancers, 40 percent of colorectal cancers, and 85–90 percent of pancreatic cancer cases. It has been thought to be an undruggable target since its discovery in 1983, but recent advancements have resulted in agents that can successfully target KRASG12C.
“The value of putting BI-0474 on opnMe is to allow global scientists to find creative uses for the compound, which may ultimately benefit cancer patients,” said Alex Waterson, research professor of pharmacology and associate director of drug discovery in the Vanderbilt Institute for Chemical Biology. “It gives researchers who may not have had access to a well-characterized G12C inhibitor before the opportunity to learn. Who knows what science will develop!”
The researchers started their work by trying to build a comprehensive attack on RAS. Preliminary KRAS research in the Fesik lab identified compounds binding at one of the protein’s two binding sites. However, the binding strength (affinity) of these molecules was very weak. This led the lab to invent a new technique to block that site, allowing them to find new chemical matter that interacts with a second site near the G12C mutation.
Uniquely, the approach that led to BI-0474 started with the optimization of a reversibly binding fragment to which an electrophile to engage the cysteine was attached. BI-0474, like other G12C-targeted compounds, works by taking advantage of the cysteine mutation. Some of the first successful attempts to target KRAS have been obtained with KRASG12C because of its sulfur, which allows researchers to make inhibitors that form a strong covalent bond with KRAS. “As the only strongly nucleophilic amino acid, working with the sulfur as a handle allowed us to take advantage of a reaction with it in a way that can’t easily be done with any other amino acid,” Waterson said. “We were trying to approach it from the reversible binder first and then add a cysteine binding function, rather than start with something that reacts first and then make it better.”
According to Boehringer Ingelheim, BI-0474 is a highly potent KRASG12C inhibitor showing a high second-order rate (kinact/KI) and low KI in mass spectrometry experiments detecting covalent protein modification of KRASG12C. It also shows in vivo biomarker modulation and in vivo efficacy in KRASG12C mutated xenograft models after intraperitoneal administration.
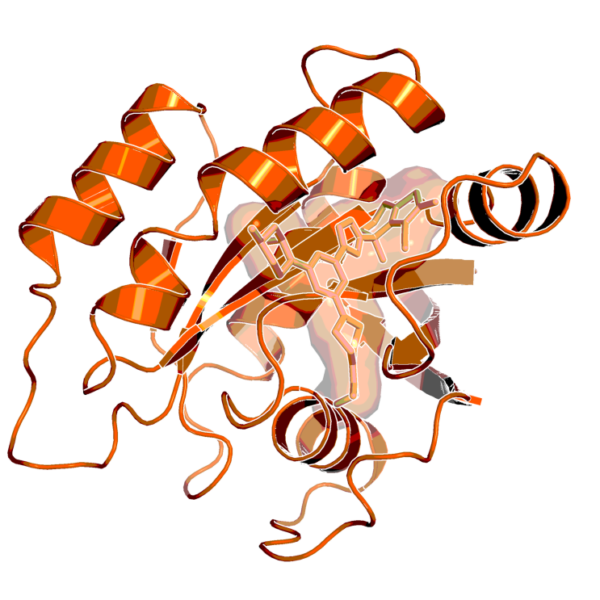
X-ray structure of the complex of BI-0474 with KRASG12C (PDB code 8AFB) (Boehringer Ingleheim)
In addition to Fesik and Waterson, Vanderbilt researchers who contributed to this work include Research Assistant Professor Jason Phan, former postdoctoral research fellows Andrew Little and Jason Abbott, and former graduate student Qi Sun.
“The joint discovery of KRASG12C inhibitor BI-0474 together with our partners from Vanderbilt University represents a remarkable success in our endeavor to combat one of the most commonly mutated oncogenetic drivers of cancer,” said Norbert Kraut, global head of cancer research of Boehringer Ingelheim. “I congratulate my colleagues as we have been able to release this well-characterized KRAS inhibitor now on our open innovation portal, opnMe.com, with the goal to share it with scientists worldwide to come up with new therapeutic strategies to transform patients’ lives.”
Support for the Vanderbilt portions of this work came from the National Institutes of Health, the Lustgarten Foundation Award, and the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences.
The Fesik lab’s research and identification of KRASG12C has been published in the Journal of Biomolecular NMR and the Journal of Medicinal Chemistry.
The previous Vanderbilt-opnMe collaboration centered around VUBI1, a compound targeting SOS1—the pacemaker of KRAS.