Summary
The material properties of mucus, such as viscosity and pH, provide information about the well-being of various organ systems. To improve accessibility to mucus sites from throughout the body and increase the validity of measurements, Vanderbilt researchers have developed a wireless millimeter-scale soft robot for direct and accurate mucus sensing throughout the body.
Addressed Need
Collection and analysis of mucus is helpful in determining if a disease or infection is present. Flexible endoscopes and capsule endoscope robots can be used to reach internal cavities and evaluate the mucus present, but their analysis is limited to visual inspection. Sample collection can help remedy this shortcoming, but it comes at the cost of altering the temperature, humidity, and pH of the sample. Furthermore, the deeper into the body and the narrower passages become, the more difficult it is to collect these samples. A method to access and directly evaluate mucus from anywhere in the body would dramatically impact the detection and diagnosis of disease.
Technology Description
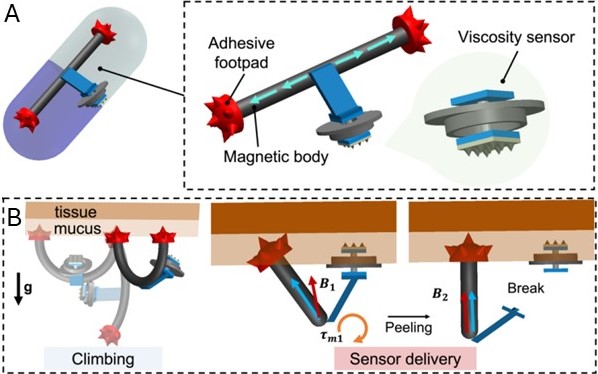
Vanderbilt engineers have developed a technology that allows clinicians to sense the material properties of mucus in situ without an invasive procedure. The robot is equipped with a viscosity sensor and two adhesive footpads for climbing (see figure). A magnetic field can be applied from outside the body to guide the robot to the desired position prior to depositing the viscosity sensor. Signals are then sent from the sensor to a receiver to temporally monitor the viscosity of the mucus.
Competitive Advantages
Miniature size enables the robot to reach small, tortuous regions of the body that are otherwise inaccessible to endoscopes. Delivery of wireless sensors in situ provides more accurate material property measurements directly from the site of interest.
Intellectual Property Status
Patents: Patent application has been filed.
Publication: Adv. Funct. Mater., 2023
Stage of Development
This technology has been validated in the lab, and we are seeking commercial partners to further develop it for clinical applications